Q&A | Covid-19
Regulating the distribution of Covid-19 vaccines with IATA
With more vaccine trials showing positive results, the supply chain – including the air cargo industry – is getting ready to deliver vaccinations on a large scale. Ilaria Grasso Macola finds out more.
To prepare for distribution, the International Air Transport Association (IATA) has released a set of guidelines the air cargo industry would need to handle, transport and distribute the Covid-19 vaccine.
IATA special cargo head Andrea Gruber explains why guidelines are needed to handle one of the world’s most complex logistics operations.

IATA special cargo head Andrea Gruber. Credit: IATA
Ilaria Grasso Macola: When did IATA start working on the guidance?
Andrea Gruber: IATA back in June released an article on Airlines magazine (the organisation’s flagship magazine) raising awareness on the upcoming challenge of a global vaccine distribution.
At that stage we raised several questions that the industry, governments and authorities would need to consider. This led us to develop the guidance document in September to list the set of considerations and awareness on large scale handling, transport and distribution of vaccines, pharmaceutical, life science and medical products.
Did IATA announce the guidance after of the positive results seen by Pfizer and Moderna?
No, but we are in close contact with pharmaceutical manufacturers, international and governmental organisations – including UNICEF, the World Food Programme and the International Civil Aviation Organisation (ICAO) – to follow the evolution of the vaccines’ development and to ensure the readiness and preparedness of the industry to the upcoming logistic challenge.
The guidance document compiles all the information available from the different segment of the supply chain.
Why was it drafted alongside partners such as ICAO and the World Trade Organisation (WTO)?
IATA’s advocacy role has led many governments and international regulatory bodies to facilitate the movement of air cargo.
The collaboration with ICAO is critical, as they represent governments who will play a large part in how this effort will play out due to their role in granting permissions for Covid-19 ad-hoc flights, converting passenger aircraft, shortening approval processes as well as developing sensible regulations and ensuring alignment with standards.
In addition, adopting trade facilitation measures can only be achieved by means of a coordinated approach with the WTO.
What are the main challenges the industry will face during the distribution phase?
The upcoming challenge for the supply chain stakeholders is to plan and execute a global network delivery mechanism for the Covid-19 vaccines, as well as medical supplies supporting the vaccines.
Stakeholders are developing various scenarios to pre-emptively establish processes and procedures in case of uncertainties, which can have a great number of implications on the supply chain planning efforts. The main challenge is certainly to respond to these uncertainties.
The first and essential element is communication along the supply chain to respond to the demand and meet the requirements once these are communicated.
There are currently 13 vaccines in phase three efficacy trials and from the information that was published, these all need different temperature requirements so the supply chain will have to adapt from ultra-cold to control-room temperature ranges.
In addition, ground-based distribution networks will have to be capable of guaranteeing temperature integrity at the destination as well as on the last-mile delivery, while the goods are secured.
What does the air cargo industry need to do to prepare?
Understand what is at stake, be informed of what is required and be able to adapt to the demand. IATA’s guidance document provides the set of considerations that the industry needs to focus on.
The air freight industry has been transporting vaccines, pharmaceuticals and medical supplies in the past and they have systems and processes in places.
However, the challenge is the scale of such transport and the requirements linked to such transport, which means the industry needs to respond to the demand rapidly and in a safe and secure environment.
How will the industry ensure that facilities are ultra-cold if some refrigerants are classified as dangerous goods?
The challenge of dry ice today is something IATA is closely working on with the authorities and the aircraft manufacturers, as dry ice’s use is regulated by the dangerous goods regulations and its quantity is limited by airline and aircraft type.
The discussions aim at ensuring that vaccines can be distributed globally while safety is guaranteed.
As mentioned before, there will be different types of vaccines requiring different temperature ranges. This means that we will see different types of supply chains depending on the storage, handling and transport capabilities.
UNICEF is for example looking at vaccines that will require conventional temperature ranges such as a +2 to +8 C.
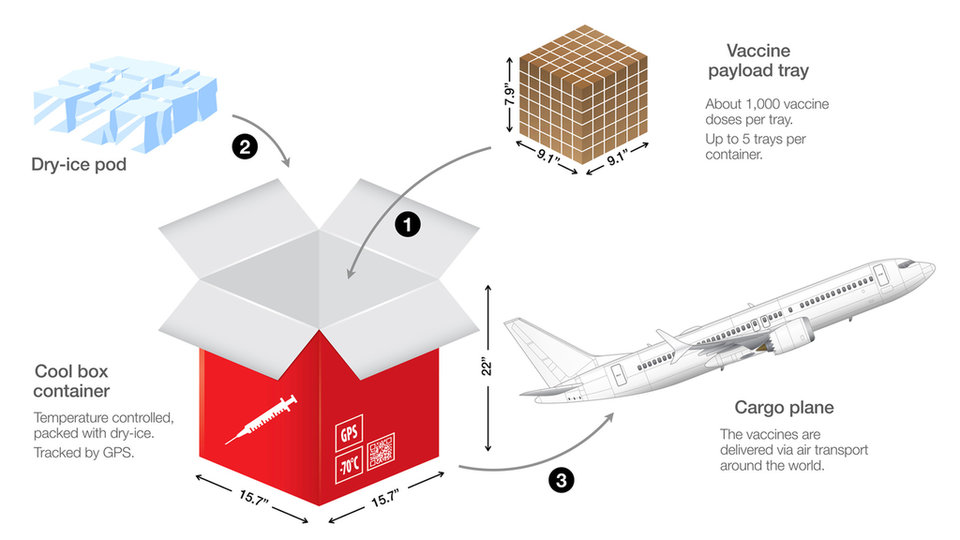
Vaccines are stored into cooled containers with dry ice and are distributed around the world.
How will the industry avoid theft and tampering?
Security concerns need to be addressed, because such large volumes of the highly valuable commodity need to be protected against tampering, theft, counterfeit and contraband.
Although the air cargo industry already has processes in place, they will need to be scaled up for the volume of vaccines.
Are more guidance documents from international organisations (such as IATA) needed if we want to have a smooth and safe distribution of vaccines on a global scale?
What is important is not [to have] more [guidance] but [to have it more] consistent.
This is the reason why IATA has released the guidance document with the contribution of the industry, international organizations and governmental agencies to align, cross-reference and provide the access to resource reference information to be best prepared to what is coming in 2021.